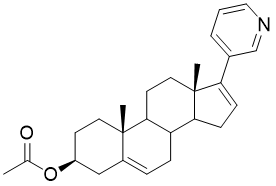
Abiraterone Acetate (CAS 154229-18-2)
Abiraterone Acetate Property Table
| Field | Value |
|---|---|
| Name | 17-(3-pyridyl)-5,16-androstadien-3β-acetate |
| CAS | 154229-18-2 |
| Synonyms | ZYTIGA, CB 7630, Abiraterone Acetate, 3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene, etc. |
| EINECS (EC#) | 620-314-7 |
| Molecular Formula | C₂₆H₃₃NO₂ |
| Molecular Weight | 391.55 |
| Appearance | White to beige powder |
| Melting Point | 127-130 °C |
| Boiling Point | 506.7 ± 50.0 °C (Predicted) |
| Density | 1.14 ± 0.1 g/cm³ (Predicted) |
| Refractive Index | 1.583 (Predicted) |
| Storage Temp. | -20°C Freezer |
| Solubility | Slightly soluble in chloroform; sparingly soluble in DMSO and methanol |
| Form | Powder |
| pKa | 5.31 ± 0.12 (Predicted) |
| Color | White to beige |
| Usage | Non-steroidal anti-inflammatory; inhibits CYP17 enzyme (17α-hydroxylase/C17,20-lyase), used for metastatic castration-resistant prostate cancer (mCRPC) and hormone-sensitive prostate cancer (HSPC). Requires prednisone co-administration. |
| Pharmacology | Selective CYP17 inhibitor; reduces testosterone levels below castration levels. |
| Clinical Use | – Indications: mCRPC, HSPC, prostate cancer bone metastasis. – Dosage: 1000 mg/day, fasting. |
| Side Effects | Common: Hypertension, hypokalemia, edema. Severe: Hepatotoxicity (monitor ALT/AST), cardiovascular events. |
| Synthesis | Synthesized from 3β-acetoxyandrosta-5,16-diene via palladium-catalyzed coupling. |
| Regulatory Status | FDA-approved (2011), EMA-approved (2011). |
| Key References | Clinical trials (Stein et al., 2014; Richards et al., 2012; Li et al., 2012). |
Additional Notes
- Synonyms: Includes brand names (e.g., ZYTIGA®) and chemical intermediates (e.g., 3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene).
- Dosage: Tablets (250 mg each), administered daily on an empty stomach.
- Pharmacokinetics:
- Bioavailability: Low (~10% orally), optimized by fasting.
- Metabolism: Rapidly converted to active metabolite abiraterone.
- Contraindications: Hypersensitivity to components, severe liver impairment.
- Drug Interactions: Avoid CYP3A4 inducers (e.g., rifampin) to prevent reduced efficacy.
- Special Populations:
- Pregnancy: Embryotoxic; contraindicated.
- Pediatrics: Not approved.
Tags: Abiraterone · CAS 154229-18-2
sale@pharma-peptides.com
请到「后台-用户-个人资料」中填写个人说明。
© 2026. All Rights Reserved. Theme By XinTheme