17α-Hydroxyprogesterone Acetate (CAS 302-23-8)
17α-Hydroxyprogesterone Acetate
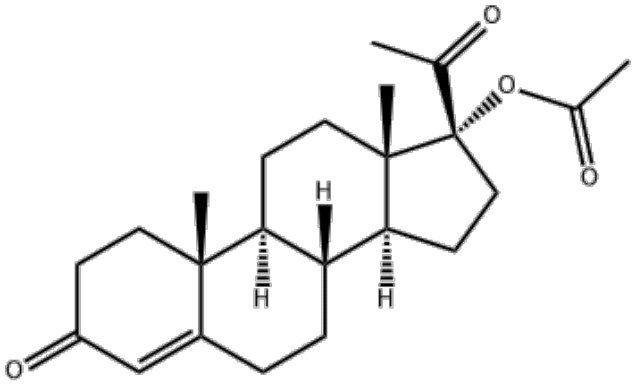
Hydroxyprogesterone Acetate Comprehensive Information Table
| Category | Details |
|---|---|
| Chemical Name | 17α-Hydroxy-4-pregnene-3,20-dione 17-acetate |
| CAS Number | 302-23-8 |
| Synonyms | 17α-Acetoxy-4-pregnene-3,20-dione, Precursor of Medroxyprogesterone Acetate (MPA), 17α-Hydroxyprogesterone 17-acetate |
| Chemical Formula | C₂₃H₃₂O₄ |
| Molecular Weight | 372.5 |
| EINECS Number | 206-119-6 |
| Melting Point | 249–250°C (lit.) |
| Boiling Point | 421.94 ± 45.0°C (predicted) |
| Density | 1.0415 ± 0.1 g/cm³ (estimated) |
| Storage Conditions | Sealed in dry, stored at 2–8°C |
| Solubility | Sparingly soluble in chloroform and ethyl acetate; slightly soluble in methanol; water solubility 1.01 mg/L (20°C) |
| Appearance | Off-white to pale yellow solid |
| Optical Activity | [α]²⁵/D +190° ±5° (c = 1 in chloroform) |
| Applications | Pharmaceutical Use: Key precursor for synthesizing medroxyprogesterone acetate (MPA) and megestrol acetate (MA). Research Use: Progesterone receptor activity studies and contraceptive drug development. |
| Quality Control | – HPLC: C18 column, methanol-water (60:40) mobile phase, UV detection at 240 nm. – Impurity Limits: Total impurities ≤0.5%. |
| Synthesis Pathway | 1. Hydroxylation: Introduction of 17α-hydroxyl group to progesterone via microbial or chemical methods. 2. Acetylation: Reaction of 17α-hydroxyprogesterone with acetic anhydride to form the acetate ester. |
| Price (Research Grade) | 30–80/gram |
| Regulatory Status | Compliant with EP and USP standards; prescription use in some countries. |
Supplementary Notes
- Structural Features:
- Contains a 17α-hydroxyl group and 3,20-dione backbone, making it a critical precursor for anabolic steroids like medroxyprogesterone acetate.
- Acetylation enhances chemical stability and prolongs biological activity.
- Synthesis Challenges:
- Requires precise stereochemical control during hydroxylation to avoid epimerization at C17.
- Solvent selection (e.g., ethanol/water) is critical for effective crystallization.
- Applications in Medicine:
- Contraception: Used as the active ingredient in long-acting injectable contraceptives (e.g., Depo-Provera®).
- Cancer Therapy: Investigated for hormone-sensitive breast and prostate cancer treatments.
- Safety Considerations:
- Irritation Risk: May cause local inflammation or allergic reactions at injection sites (risk statements R36/37/38).
- Long-Term Use: High doses may suppress bone density; calcium metabolism monitoring is recommended.
For detailed toxicological data or formulation protocols, consult specialized pharmacological references.
Tags: CAS 302-23-8 · Hydroxyprogesterone
sale@pharma-peptides.com
请到「后台-用户-个人资料」中填写个人说明。
© 2026. All Rights Reserved. Theme By XinTheme